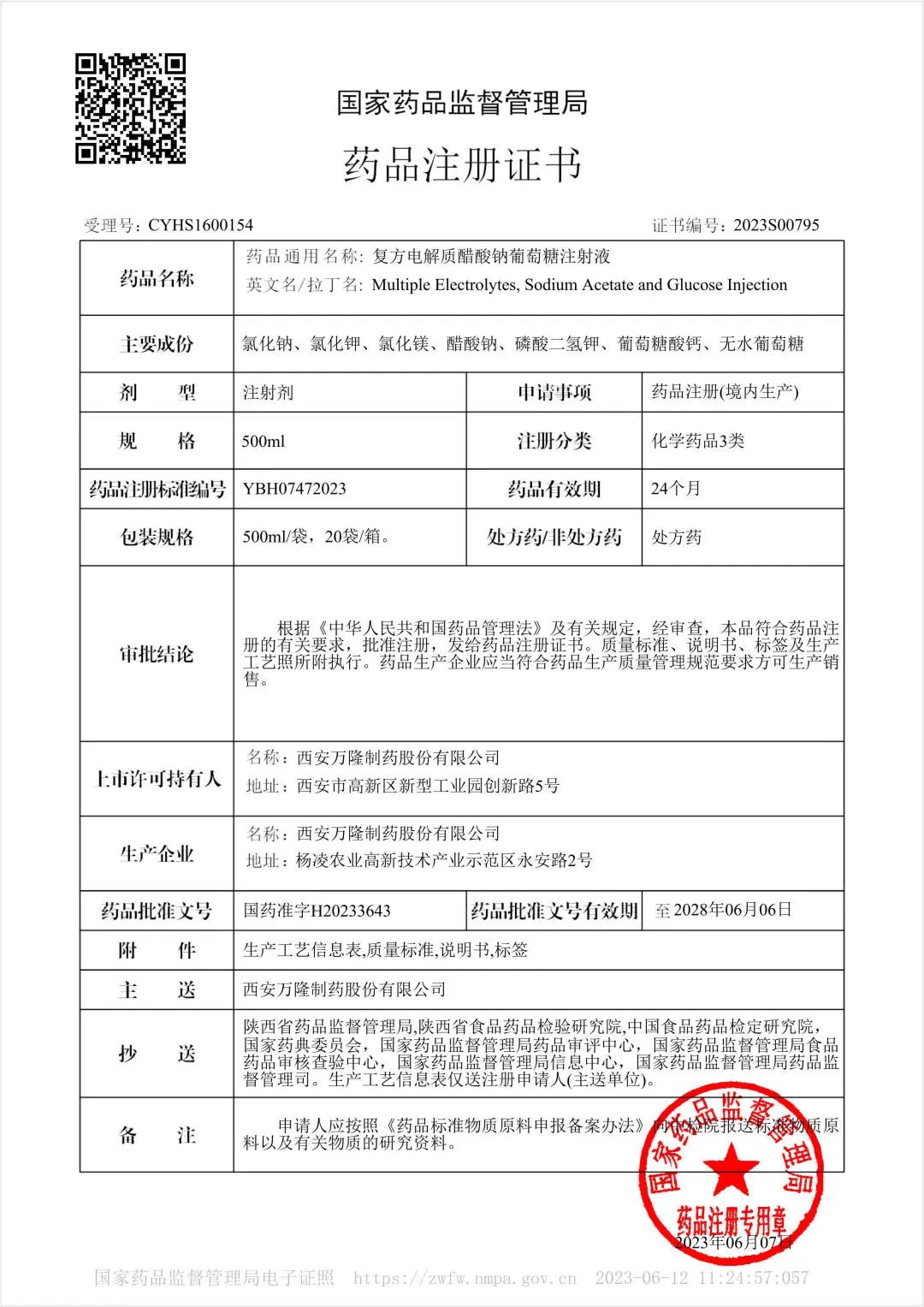
On June 7, 2023, our product "Multiple electrolytes, sodium acetate and glucose injection" was approved by National Medical Products Administration. It belongs to registration classification 3, in other words, it is regarded as the evaluation of consistency.
The effective components in Multiple electrolytes, sodium acetate and glucose injection are sodium chloride, potassium chloride, magnesium chloride, sodium acetate, potassium dihydrogen phosphate, calcium gluconate and anhydrous glucose. Strengthen is 500mL/bag, and expiry date is 24 months. It is a new generation of crystal liquid which integrates the three effects of replenishing liquid, balancing electrolyte and high efficiency energy supply. It is rich in essential electrolyte ions, regulating metabolic disorders; 10% glucose content, phosphorus ion to accelerate glycolysis, efficient energy supply. Acetic acid ions replace lactic acid, maintain liver and kidney function, safe and reliable. Clinically, it can be widely used in surgery, ICU, anesthesia, tumor, digestion, burn and other departments.
Xi'an Wanlong Pharmaceutica always regards quality and safety as the primary task of the enterprise, and at the same time, fully implements the pharmaceutical consistency evaluation work of the "12th Five-Year Plan of National Medical Safety". We insist on developing and manufacturing first-class products, earnestly fulfill the duties of pharmaceutical industry, and actively contribute to the society. The successful approval of Multiple electrolytes, sodium acetate and glucose injection is also the embodiment of enterprise spirit. Xi'an Wanlong Pharmaceutical will continue to increase the investment in scientific and technological innovation, continuous development and innovation, to provide patients with reliable quality, significant curative effect, safe and secure good medicine, and continue to serve the society with high-quality products!